What is U bend brass Tube Heat Exchanger?
U bend brass Tube Heat Exchanger Introduction:
U-shaped brass tube heat exchanger is a kind of shell and tube heat exchanger, which consists of tube sheet, shell, tube bundle and other parts.
Under the same diameter, the U-shaped tube heat exchanger has a large heat exchange area; it has a simple and compact structure, high sealing performance, convenient maintenance and cleaning, low metal consumption under high temperature and high pressure, and low cost; the U-shaped tube heat exchanger has only one tube sheet, good thermal compensation performance, strong pressure bearing capacity, and is suitable for operation under high temperature and high pressure conditions.
U bend brass Tube Heat Exchanger Structure:
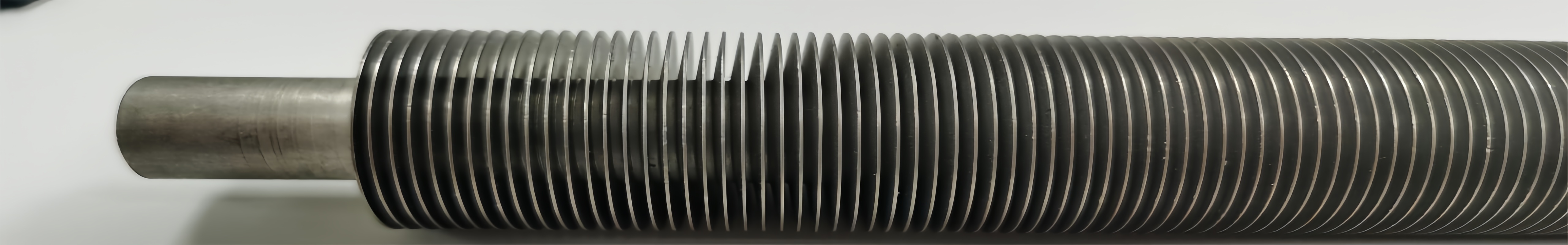
The main structure of the U-tube heat exchanger includes the tube box, cylinder, head, heat exchange tube, connecting pipe, baffle, anti-impact plate and guide tube, anti-short circuit structure, support and other accessories of the shell and tube.
Heat exchange tube:
►1. Ordinary heat exchange tubes
The heat exchange tubes used for heat transfer usually use higher-grade cold-drawn heat exchange tubes and ordinary-grade cold-drawn heat exchange tubes. The former is suitable for heat transfer without phase change and easy vibration occasions, and the latter is suitable for reboiling, condensation heat transfer and general occasions without vibration.
The forms of heat exchange tubes are various. Smooth tubes are the traditional form. Because of their advantages such as easy manufacturing and low unit length cost, they are more common in current applications. The tubes should be able to withstand a certain temperature difference and stress. When the tube-side and shell-side fluids are corrosive, the tubes should also have corrosion resistance.
Recommended length:
The recommended length of heat exchange tubes is as follows: 1.0m, 1.5m, 2.0m, 2.5m, 3.0m, 4.5m, 6.0m, 7.5m, 9.0m, 12.0m. For a certain heat exchange area, longer heat exchange tubes are more economical, so the heat exchangers in engineering are generally slender structures. However, if the heat exchange tubes are too long, it will be detrimental to the installation and maintenance of the heat exchanger.
Available material:
The materials of heat exchange tubes come from a wide range of sources, including carbon steel, stainless steel, aluminum, copper and its alloys, copper-nickel alloys, nickel alloys and other special materials. In addition to using a single material to manufacture heat exchange tubes, composite tubes are also often used to meet production requirements.
►2. High-efficiency heat exchange tubes
In order to simultaneously expand the effective heat transfer area inside and outside the tube or enhance heat transfer, and to greatly improve the heat transfer coefficient of the tube, the inner and outer surfaces of the heat exchange tube are rolled into various surface shapes, or a turbulent element is inserted into the tube to simultaneously generate turbulence in the fluid inside and outside the tube, thereby improving the performance of the heat exchange tube. A variety of high-efficiency heat exchange tubes have been developed.
Common types:
Most high-efficiency heat exchange tubes have the function of enhancing heat transfer both inside and outside the tube. According to different working conditions, different combinations of high-efficiency heat exchange tubes and new tube bundle supports can achieve relatively ideal heat transfer effects.
U-shaped tube production process:
Straight tube – 100% flaw detection before bending – Reliable bending machine and precision bending mold – Electrical heating stress relief annealing of U-shaped part after bending – 100% water pressure after bending.
The heat exchange tubes of the U-shaped brass tube heat exchanger are mainly made of brass alloy.
Brass grades:
ASTM B111 C68700
ASTM B111 C44300
Brass is an alloy composed of copper and zinc. If it is only composed of copper and zinc, it is called ordinary brass. Brass is often used to make valves, water pipes, air conditioner internal and external unit connecting pipes and radiators. If it is a variety of alloys composed of more than two elements, it is called special brass. For example, copper alloys composed of lead, tin, manganese, nickel, iron and silicon. Special brass is also called special brass. It has high strength, high hardness and strong chemical corrosion resistance. The mechanical properties of cutting processing are also outstanding. Brass has strong wear resistance. Seamless copper tubes drawn from brass are soft and have strong wear resistance. Brass seamless tubes can be used for heat exchangers and condensers, low-temperature pipelines, and submarine transport pipes. Manufacture of sheets, strips, bars, pipes, casting parts, etc. The copper content is 62% to 68%, with strong plasticity, and manufacturing pressure-resistant equipment. According to the different types of alloy elements contained in brass, brass is divided into ordinary brass and special brass. Brass used for pressure processing is called deformed brass. Brass is a copper alloy with zinc as the main added element. It has a beautiful yellow color and is generally called brass. Copper-zinc binary alloy is called ordinary brass or simple brass. Brass with more than ternary content is called special brass or complex brass. Brass alloys with zinc content less than 36% are composed of solid solutions and have good cold working properties. For example, brass with zinc content of 30% is often used to make shells, commonly known as shell brass or 7-3 brass. Brass alloys with zinc content between 36% and 42% are composed of and solid solutions. The most commonly used is 6-4 brass with zinc content of 40%. In order to improve the performance of ordinary brass, other elements such as aluminum, nickel, manganese, tin, silicon, lead, etc. are often added. Aluminum can improve the strength, hardness and corrosion resistance of brass, but reduce plasticity, making it suitable for sea-going ship condensers and other corrosion-resistant parts. Tin can improve the strength and corrosion resistance of brass to seawater, so it is called naval brass, which is used for ship thermal equipment and propellers. Lead can improve the cutting performance of brass; this easy-to-cut brass is often used as watch parts. Brass castings are often used to make valves and pipe fittings.
Main performance:
►Room temperature structure
Ordinary brass is a copper-zinc binary alloy, and its zinc content varies widely, so its room temperature structure is also very different. According to the Cu-Zn binary phase diagram, there are three types of room temperature structures of brass: brass with a zinc content of less than 35% has a microstructure composed of a single-phase α solid solution at room temperature, called α brass; brass with a zinc content of 36% to 46% has a microstructure composed of two phases (α+β) at room temperature, called (α+β) brass (two-phase brass); brass with a zinc content of more than 46% to 50% has a microstructure composed only of β phase at room temperature, called β brass.
►Pressure processing performance
α single-phase brass (from H96 to H65) has good plasticity and can withstand hot and cold processing, but α single-phase brass is prone to medium-temperature brittleness during hot processing such as forging. The specific temperature range varies with the Zn content, generally between 200 and 700°C. Therefore, the temperature during hot processing should be higher than 700°C. The main reason for the medium-temperature brittleness zone of single-phase α brass is that there are two ordered compounds, Cu3Zn and Cu9Zn, in the α phase region of the Cu-Zn alloy system. When heated at medium and low temperatures, an ordered transformation occurs, making the alloy brittle; in addition, there are trace amounts of lead and bismuth harmful impurities in the alloy that form low-melting point eutectic films with copper and distribute on the grain boundaries, causing intergranular cracks during hot processing. Practice has shown that adding trace amounts of cerium can effectively eliminate medium-temperature brittleness.
In addition to the α phase with good plasticity, the two-phase brass (from H63 to H59) also has a β solid solution based on the electronic compound CuZn in the alloy structure. The β phase has high plasticity at high temperatures, while the β′ phase (ordered solid solution) at low temperatures is hard and brittle. Therefore, (α+β) brass should be forged in a hot state. β brass with a zinc content greater than 46% to 50% cannot be press-processed due to its hard and brittle properties.
►Mechanical properties
Due to the different zinc content in brass, the mechanical properties of brass are also different. The mechanical properties of brass vary with the zinc content. For α brass, as the zinc content increases, σb and δ continue to increase. For (α+β) brass, the room temperature strength continues to increase before the zinc content increases to about 45%. If the zinc content is further increased, the strength will drop sharply due to the appearance of the more brittle r phase (solid solution based on Cu5Zn8 compounds) in the alloy structure. The room temperature plasticity of (α+β) brass always decreases with the increase of zinc content. Therefore, copper-zinc alloys with a zinc content of more than 45% have no practical value.
Ordinary brass has a wide range of uses, such as water tank belts, water supply and drainage pipes, medals, bellows, serpentine pipes, condensers, shells, and various complex-shaped punching products, small hardware, etc. As the zinc content increases from H63 to H59, they can all withstand hot processing well and are mostly used in various parts of machinery and electrical appliances, stampings and musical instruments.
In order to improve the corrosion resistance, strength, hardness and machinability of brass, a small amount of tin, aluminum, manganese, iron, silicon, nickel, lead and other elements are added to the copper-zinc alloy (generally 1% to 2%, a few up to 3% to 4%, and very rarely up to 5% to 6%) to form a ternary, quaternary or even quinary alloy, which is complex brass, also known as special brass.
►Zinc equivalent coefficient
The structure of complex brass can be calculated based on the "zinc equivalent coefficient" of the elements added to the brass. Because adding a small amount of other alloying elements to a copper-zinc alloy usually only moves the α/(α+β) phase region in the Cu-Zn state diagram to the left or right. Therefore, the structure of special brass is usually equivalent to the structure of ordinary brass with increased or reduced zinc content. For example, the structure after adding 1% silicon to Cu-Zn alloy is equivalent to the alloy structure adding 10% zinc to Cu-Zn alloy. So the "zinc equivalent" of silicon is 10. The "zinc equivalent coefficient" of silicon is the largest, which causes the α/(α+β) phase boundary in the Cu-Zn system to significantly move to the copper side, that is, the α phase area is strongly reduced. The "zinc equivalent coefficient" of nickel is negative, which means the α phase area is expanded.
The α phase and β phase in special brass are multi-complex solid solutions, and their strengthening effect is large, while the α and β phases in ordinary brass are simple Cu-Zn solid solutions, and their strengthening effect is low. Although the zinc equivalents are equivalent, the properties of multicomponent solid solutions are different from those of simple binary solid solutions. Therefore, a small amount of multi-component strengthening is a way to improve the properties of alloys.
Main classification:
►Lead brass
Lead is actually insoluble in brass and is distributed on the grain boundaries in the form of free particles. There are two types of lead brass according to its structure: α and (α+β). α lead brass has a large harmful effect of lead and has very low high temperature plasticity, so it can only be cold deformed or hot extruded. (α+β) lead brass has good plasticity at high temperatures and can be forged.
►Tin brass
Adding tin to brass can significantly improve the heat resistance of the alloy, especially the ability to resist seawater corrosion, so tin brass is known as "naval brass".
Tin can dissolve in copper-based solid solution and play a role in solid solution strengthening. However, with the increase of tin content, brittle r phase (CuZnSn compound) will appear in the alloy, which is not conducive to the plastic deformation of the alloy, so the tin content of tin brass is generally in the range of 0.5% to 1.5%.
Commonly used tin brass are HSn70-1, HSn62-1, HSn60-1, etc. The former is an α alloy with high plasticity and can be processed by cold and hot pressure. The latter two grades of alloys have a (α+β) two-phase structure and often have a small amount of r phase. The room temperature plasticity is not high and can only be deformed in the hot state.
►Manganese brass
Manganese has greater solubility in solid brass. Adding 1% to 4% manganese to brass can significantly improve the strength and corrosion resistance of the alloy without reducing its plasticity.
Manganese brass has an (α+β) structure, and HMn58-2 is commonly used. The pressure processing performance in cold and hot states is quite good.
►iron brass
In iron brass, iron precipitates as particles of iron-rich phase, which serve as crystal nuclei to refine the grains and prevent recrystallized grains from growing, thereby improving the mechanical properties and process performance of the alloy. The iron content in iron brass is usually less than 1.5%, its structure is (α+β), it has high strength and toughness, has good plasticity at high temperatures, and can also be deformed in the cold state. The commonly used grade is Hfe59-1-1.
►Nickel brass
Nickel and copper can form a continuous solid solution and significantly expand the α phase area. Adding nickel to brass can significantly improve the corrosion resistance of brass in the atmosphere and seawater. Nickel also increases the recrystallization temperature of brass, promoting the formation of finer grains.
HNi65-5 nickel brass has a single-phase α structure, has good plasticity at room temperature, and can also be deformed in a hot state. However, the content of impurity lead must be strictly controlled, otherwise the hot working performance of the alloy will be seriously deteriorated.
Purity measurement
The purity of brass can be measured using the Archimedean principle. The volume and mass of the sample are measured, and then the proportion of copper in the brass can be calculated based on the density of copper and the density of zinc.
►Ordinary brass
It is an alloy composed of copper and zinc.
When the zinc content is less than 35%, zinc can dissolve in copper to form a single phase α, called single-phase brass, which has good plasticity and is suitable for hot and cold pressure processing.
When the zinc content is 36%~46%, there is an α single phase and a β solid solution based on copper and zinc, called a dual-phase brass. The β phase reduces the plasticity of brass and increases the tensile strength, which is only suitable for hot pressure processing.
If the mass fraction of zinc continues to increase, the tensile strength decreases and has no use value.
The code is represented by "H + number", H represents brass, and the number represents the mass fraction of copper.
For example, H68 represents brass with a copper content of 68% and a zinc content of 32%. For cast brass, the letter "Z" is added before the code, such as ZH62.
For example, ZCuZnzn38 represents cast brass with a zinc content of 38% and the remainder of copper.
H90 and H80 are single-phase brass, golden yellow.
H59 is a duplex brass, widely used in electrical structural parts, such as bolts, nuts, washers, springs, etc.
In general, single-phase brass is used for cold deformation processing, and duplex brass is used for hot deformation processing.
►Special brass
The multi-element alloy composed of other alloying elements added to ordinary brass is called special brass. Commonly added elements include lead, tin, aluminum, etc., which can be called lead brass, tin brass, and aluminum brass accordingly. The purpose of adding alloying elements. It is mainly to improve tensile strength and processability.
Code: "H + main added element symbol (except zinc) + copper mass fraction + main added element mass fraction + other element mass fraction".
For example: HPb59-1 means lead brass with a mass fraction of 59% copper, a mass fraction of 1% of the main added element lead, and the remainder is zinc.
Heat treatment specifications:
Hot working temperature 750~830℃; annealing temperature 520~650℃; low temperature annealing temperature to eliminate internal stress 260~270℃.
Environmentally friendly brass C26000 C2600 has excellent plasticity, high strength, good machinability, welding, good corrosion resistance, heat exchangers, papermaking tubes, machinery, electronic parts.
Specifications (mm): Specifications: Thickness: 0.01-2.0mm, Width: 2-600mm;
Hardness: O, 1/2H, 3/4H, H, EH, SH, etc.;
Applicable standards: GB, JISH, DIN, ASTM, EN;
Features: Excellent cutting performance, suitable for high-precision parts processed by automatic lathes and CNC lathes.